
We are pleased to be able to offer NeuroStar Advanced Therapy TMS. Transcranial Magnetic Stimulation Therapy is one of the most technologically advanced depression treatments available. This non-invasive, outpatient therapy is FDA cleared and has helped countless patients who have not received adequate results from medication.
NeuroStar Advanced Therapy is FDA cleared for the treatment of depression, OCD, and anxious depression.

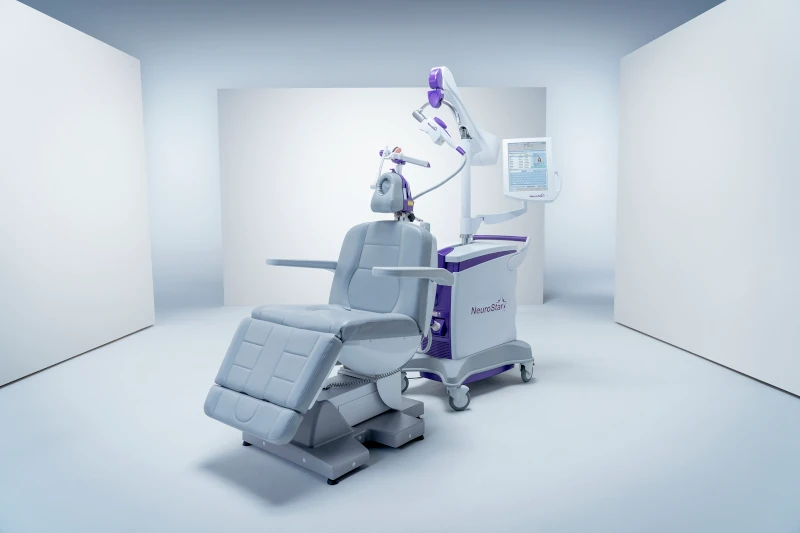

NeuroStar Advanced Therapy is Transcranial Magnetic Stimulation (TMS), a non-drug treatment for major depression. During the NeuroStar Advanced Therapy treatment sessions, a magnet that is similar in strength to one that is utilized in a MRI (magnetic resonance imaging)machine, is used to stimulate the nerve cells in the brain area that are thought to control a person’s mood.
These magnetic pulses can have a positive effect on an individual’s brain transmitter levels which can make long-term remission possible.
A safe, effective depression treatment without the common side effects of medication.
Most insurance providers now offer coverage for NeuroStar Advanced Therapy TMS treatment.
A non-invasive in-office treatment that reawakens the connections in your brain.
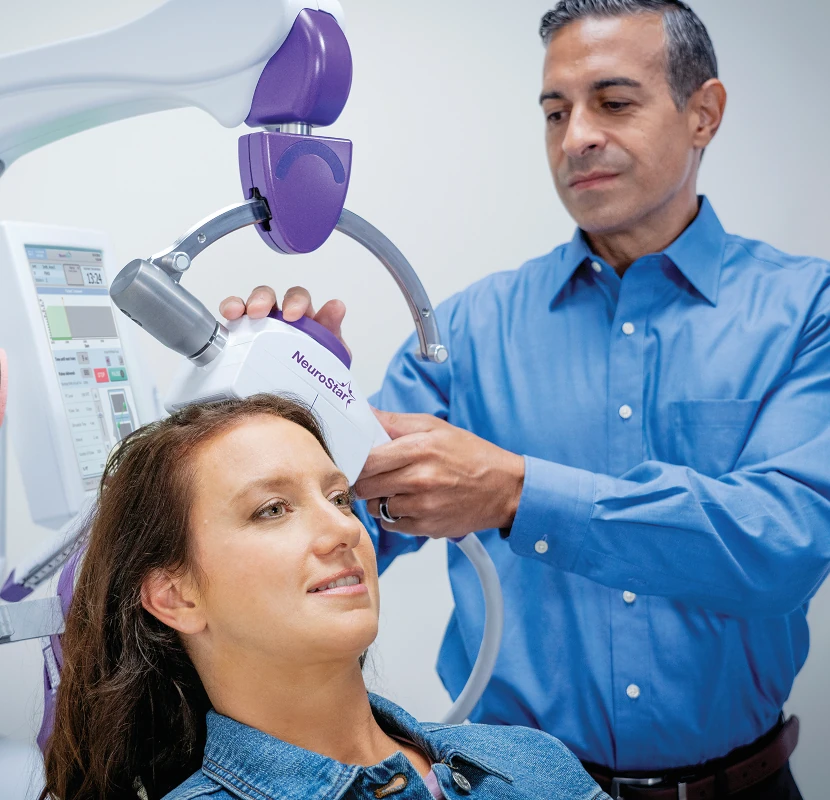
NeuroStar Advanced Therapy is Transcranial Magnetic Stimulation (TMS), a non-drug treatment for major depression. NeuroStar Advanced Therapy uses focused magnetic pulses, similar to an MRI, to reignite dormant synapses in the brain and help your brain function the way it was meant to.


Psychiatrist

TMS Technician
TMS stands for transcranial magnetic stimulation. It is used to treat depression by stimulating the brain non-invasively using electromagnetic fields, similar to those produced by an MRI machine. During NeuroStar Advanced Therapy TMS, a magnetic field is administered in very short pulses to the part of the brain that research has demonstrated to be associated with depression. The typical initial course of treatment is about 19-37 minutes daily over 7 weeks.
The NeuroStar Advanced Therapy TMS system uses short pulses of magnetic fields to stimulate the area of the brain that is thought to function abnormally in patients with depression. The magnetic field produces an electric current in the brain that stimulates the brain cells (neurons). This results in changes that are thought to be beneficial in the treatment of depression.
It usually takes time for healthcare insurers to establish coverage policies for newly approved treatments such as NeuroStar Advanced Therapy TMS. However, many commercial and Medicare plans have recognized the effectiveness of treating depression with NeuroStar Advanced Therapy TMS and now cover NeuroStar Advanced Therapy TMS as part of their plans. See here for a full list of insurance plans that cover TMS.
NeuroStar Advanced Therapy TMS is non-systemic (does not circulate in the blood throughout the body), so it does not have side effects such as weight gain, sexual dysfunction, nausea, dry mouth, sedation, etc. The most common side effects reported during clinical trials were headache and scalp discomfort – generally mild to moderate – occurring less frequently after the first week of treatment.
No, the most common side effect related to treatment was scalp discomfort during treatment sessions. This side effect was generally mild to moderate, and occurred less frequently after the first week of treatment.
If necessary, you can treat this discomfort with an over-the-counter analgesic. If these side effects persist, your doctor can temporarily reduce the strength of the magnetic field pulses being administered in order to make treatment more comfortable.
Less than 5% of patients treated with NeuroStar Advanced TMS Therapy discontinued treatment due to side effects.